Submitted by Vicky Yee K Reid on Mon, 22/07/2024 - 12:02
Can we apply engineering biology principles to develop kinder, more effective treatments for cancer? From innovative CAR-T therapies, to new tools for drug discovery and potential new treatments. Learn more about the University of Cambridge researchers exploring precision medicines for cancer.
Improving CAR-T Immunotherapies
Dr Mike Chapman, Dr Georgina Anderson and Dr Ieuan Walker
Mike Chapman's lab works on understanding and treating multiple myeloma (MM). This is a particularly aggressive form of blood cancer. One promising treatment for MM is CAR-T therapy. It involves editing a patient's immune cells to target cancer cells. The cells are then put back into the patient's bloodstream to find and attack cancer cells. One problem with using CAR-T therapy is that sometimes the edited cells also recognise and attack healthy tissue. This can cause unwanted side effects. Postdoctoral Fellow Dr Georgina Anderson and PhD student Dr Ieuan Walker are working on ways to solve this problem.
Their work uses 'AND' and 'NOT' logic gates and takes complementary approaches. With AND gates, the cells have to recognise two parts of the cancer cell, making it less likely that they will attack healthy cells. With NOT gates, the cells recognise healthy tissue and know not to attack it. Both methods aim to reduce side effects and improve treatment efficiency. Both researchers are currently testing their systems on new combinations of targets. They are also sharing their methods to help others develop more specific treatments.
CAR-T cells using AND of NOT logic gates could help reduce treatment side effects.
New Antibody Combinations for Drug Discovery
Prof Mark Howarth
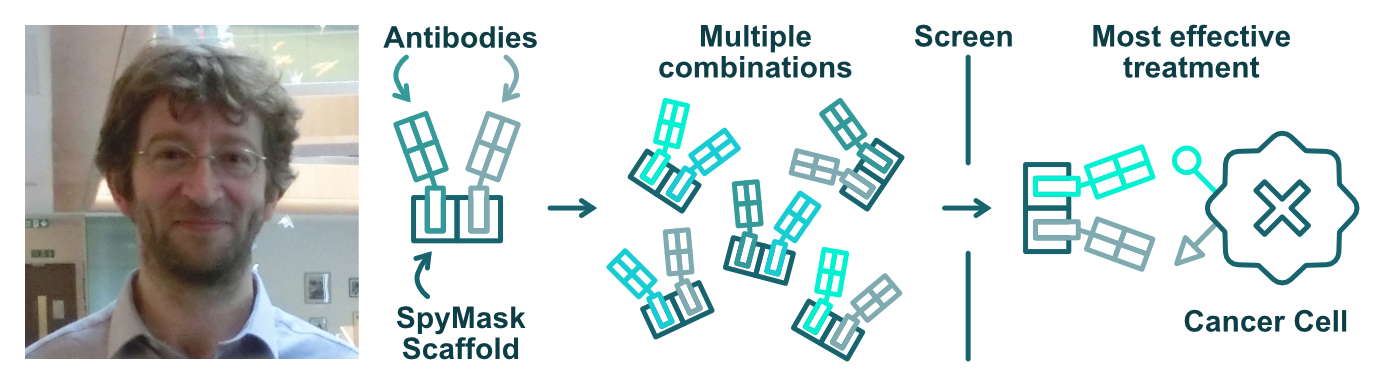
Mark Howarth's lab works on engineering proteins for new therapies and vaccines. The lab has developed the SpyTag/SpyCatcher system. This is a tool that sticks proteins together, allowing them to work in new ways. One application of this technology is to create ‘bispecific antibodies’. These are antibodies that recoginse more than one part of a cell. Engineered antibodies are very useful for treating cancer, as they can recognise specific parts of cancer cells. Bispecific antibodies can be even more useful, as they recognise multiple parts of a cell and can have multiple antitumor effects.
Recent work in the Howarth lab has developed a system called SpyMask. This process allows them to quickly assemble, screen and purify different antibody combinations and conformations. The group have now tested their system on a common cancer target (HER2). In the future, they believe that this new tool could help to accelerate and fine-tune cancer drug discovery.
The SpyMask method can produce and screen new antibody combinations for better treatments.
Nanobodies to Correct Cancer Mutations
Prof Laura Itzhaki
Laura Itzhaki's lab works on a class of proteins known-as tandem-repeat proteins. These proteins are made up of repeated structural motifs that form elongated spring-like structures. They are important as they are often altered in human diseases.Because of their repetitive structures, these proteins are easy to modify and redesign. Laura’s group is using redesigned tandem-repeat proteins in several ways. This includes engineering them to help get rid of disease-causing proteins. They are also designing small molecules, peptides and proteins to target tandem-repeat proteins that cause diseases.
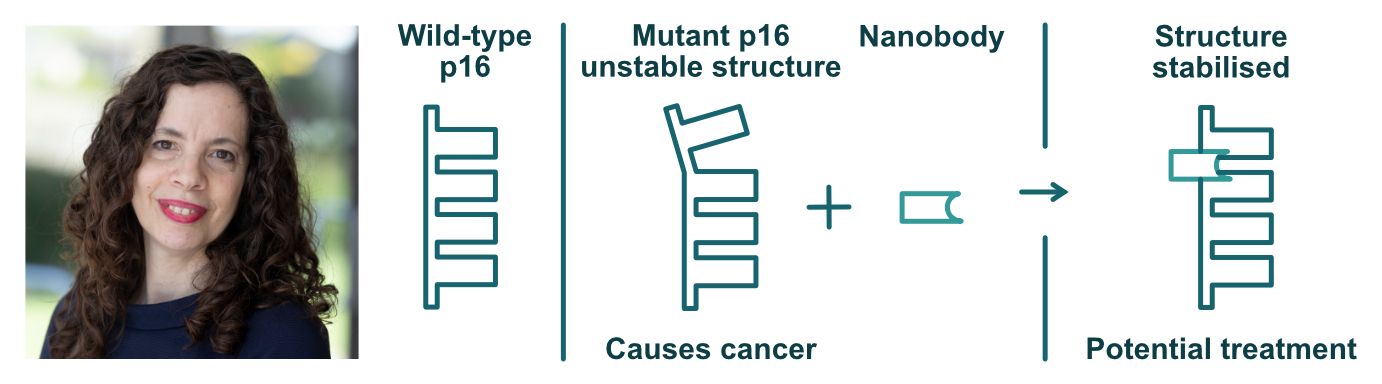
For example, they have developed antibody-like molecules called nanobodies that can correct the malfunction of a tandem-repeat protein called p16. Mutations in p16 can cause changes to its shape and structure. These changes can sometimes cause cancer. The Itzhaki lab has shown that nanobodies can correct these changes and stabilise the structure of p16. They hope that these antibodies could be used to help treat cancer. Laura's lab has also recently been awarded to new grants (BBSRC Engineering Biology Mission Award and a Cambridge Isaac Newton Trust Strategic Grant) which will help to establish protein library screening facilities. These new facilities will allow faster development and screening of potential new treatments, and bolster engineering biology research in Cambridge.
A nanobody against the tandem repeat protein p16 could help to stabilise its structure and correct for cancer-causing mutations.
These examples show how engineering biology is paving the way for new and improved cancer therapies. Researchers at the University of Cambridge are at the forefront of these exciting developments, and are continuing to find new ways to develop kinder, more effective treatments.
Learn More
Learn more about how researchers at the University of Cambridge are changing the story of cancer and explore the engineering biology researchers involved in biomedical research.
Further Reading
How is engineering biology transforming healthcare?
- Applications of synthetic biology in medical and pharmaceutical fields. Yan X, Liu X, Zhao C, Chen GQ. Signal Transduct Target Ther. (2023) 11;8(1):199. doi: 10.1038/s41392-023-01440-5
- Plant-based biopharmaceutical engineering. Eidenberger L, Kogelmann B, Steinkellner H. Nat Rev Bioeng. (2023) 1(6):426-439. doi: 10.1038/s44222-023-00044-6
- Synthetic biomarkers: a twenty-first century path to early cancer detection. Kwong, G.A., Ghosh, S., Gamboa, L. et al. Nat Rev Cancer (2021) 21, 655–668. doi: 10.1038/s41568-021-00389-3
- Synthetic biology and personalized medicine. Jain KK. Med Princ Pract. (2013) 22(3):209-19. doi: 10.1159/000341794
How is engineering biology transforming cancer treatment?
- Engineering advanced cancer therapies with synthetic biology. Wu, MR., Jusiak, B. & Lu, T. Nat Rev Cancer (2019) 19, 187–195. doi: 10.1038/s41568-019-0121-0
- CAR T-cell Therapy. Cancer Research UK. Last reviewed: 28 Jun 2024.
- Reprogramming Synthetic Cells for Targeted Cancer Therapy. Lim B, Yin Y, Ye H, Cui Z, Papachristodoulou A, Huang WE. (2022) ACS Synth Biol. 18;11(3):1349-1360. doi: 10.1021/acssynbio.1c00631
AND and NOT gated CAR-T Cell Therapies
- CAR T-cell Therapy. Cancer Research UK. Last reviewed: 28 Jun 2024.
- To go or not to go? Biological logic gating engineered T cells. Abbott RC, Hughes-Parry HE, Jenkins MR. Journal for ImmunoTherapy of Cancer (2022) 10:e004185. doi: 10.1136/jitc-2021-004185
- And-Gate CAR T-Cells to Improve Tumour Specificity and Targeting of Low-Expression Antigens in Multiple Myeloma. Georgina S.F. Anderson, Ieuan Walker, James P Roy, Michael A. Chapman. Blood (2023) 142 (Supplement 1): 751. doi: 10.1182/blood-2023-187418
- Targeting myeloma essential genes using NOT Gated CAR T-cells, a computational approach. Walker IG, Roy JP, Anderson GSF, Guerrero Lopez J, Chapman MA. Leukemia. (2024) Apr 30. doi: 10.1038/s41375-024-02247-1
p16 and Making use of Tandem-Repeat Proteins
- Role of the p16 tumor suppressor gene in cancer. Liggett WH Jr, Sidransky D. J Clin Oncol. (1998) Mar;16(3):1197-206. doi: 10.1200/JCO.1998.16.3.1197
- Nanobodies restore stability to cancer-associated mutants of tumor suppressor protein p16INK4a. Owen Burbidge, Martyna W. Pastok, Samantha L. Hodder, Grasilda Zenkevičiūtė, Martin E. M. Noble, Jane A. Endicott, Laura Itzhaki. bioRxiv (2021) 2021.07.01.450670; doi: 10.1101/2021.07.01.450670
- Engineering mono- and multi-valent inhibitors on a modular scaffold. Diamante A, Chaturbedy PK, Rowling PJE, Kumita JR, Eapen RS, McLaughlin SH, de la Roche M, Perez-Riba A, Itzhaki LS. Chem Sci. (2020) Dec 17;12(3):880-895. doi: 10.1039/d0sc03175e
- Consensus tetratricopeptide repeat proteins are complex superhelical nanosprings. Marie Synakewicz, Rohan S. Eapen, Albert Perez-Riba, Daniela Bauer, Andreas Weißl, Gerhard Fischer, Marko Hyvönen, Matthias Rief, Laura S. Itzhaki, Johannes Stigler. bioRxiv (2021) 2021.03.27.437344; doi: 10.1101/2021.03.27.437344
- Tandem-repeat proteins introduce tuneable properties to engineered biomolecular condensates. Tin Long Chris Ng, Mateo P. Hoare, M. Julia Maristany, Ellis J. Wilde, Tomas Sneideris, Jan Huertas, Belinda K. Agbetiameh, Mona Furukawa, Jerelle A. Joseph, Tuomas P.J. Knowles, Rosana Collepardo-Guevara, Laura S. Itzhaki, Janet R. Kumita. bioRxiv (2024) 2024.04.16.589709; doi: 10.1101/2024.04.16.589709
SpyTag, SpyMask and Bispecific Antibodies
- Monoclonal Antibodies (MABs). Cancer Research UK. Last reviewed: 08 Jan 2021
- Bispecific Antibodies: An Area of Research and Clinical Applications. USA Food and Drug Administration. Last reviewed: 14 Feb 2024
- Peptide tag forming a rapid covalent bond to a protein, through engineering a bacterial adhesin. Zakeri B, Fierer JO, Celik E, Chittock EC, Schwarz-Linek U, Moy VT, Howarth M. Proc Natl Acad Sci (2012) Mar 20;109(12):E690-7. doi: 10.1073/pnas.1115485109
- Catching a SPY: Using the SpyCatcher-SpyTag and Related Systems for Labeling and Localizing Bacterial Proteins. Hatlem D, Trunk T, Linke D, Leo JC. Int J Mol Sci. (2019) Apr 30;20(9):2129. doi: 10.3390/ijms20092129
- SpyMask enables combinatorial assembly of bispecific binders. Driscoll, C.L., Keeble, A.H. & Howarth, M.R. Nat Commun (2014) 15, 2403. doi: 10.1038/s41467-024-46599-9
Author Information
Credits & Acknowledgements
Many thanks to Dr Mike Chapman, Dr Georgina Anderson, Ieuan Walker, Dr Mark Howarth and Laura Itzhaki for sharing their work with us.
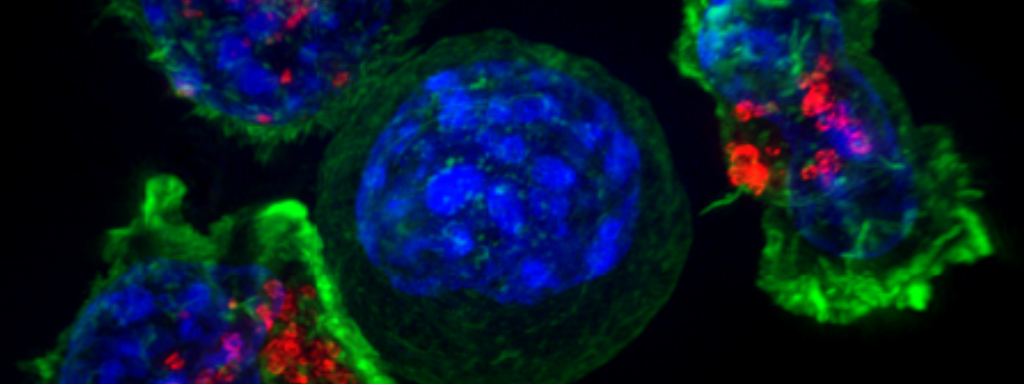
Headline image: Flouresence microscopy image of a cancer cell surrounded by killer T cells, illustrating how CAR-T therapies work. Image courtesy of the NIH Image library: Alex Ritter, Jennifer Lippincott Schwartz and Gillian Griffiths.