Submitted by Vicky Yee K Reid on Tue, 10/12/2024 - 15:42
Researchers in the Hollfelder group are using novel ultrahigh-throughput screening techniques to discover and improve new biocatalysts for plastic degradation as part of a new UKRI-funded mission hub.
The P3EB mission hub
The Preventing Plastic Pollution with Engineering Biology (P3EB) Hub is one of 6 major UKRI-funded Mission Hubs, designed to tackle major global challenges and transform our health and environment. The Hub will see researchers at the University of Cambridge collaborating with leading scientists across the country to discover and engineer enzymes to more efficiently degrade some of the most widely used and environmentally problematic plastics: PET, polyurethanes, polycarbonates and nylons. The team in Cambridge is led by Prof. Florian Hollfelder and will focus on the directed evolution and high-throughput screening of these novel, plastic degrading enzymes.
Directed evolution can make new and more efficient enzymes
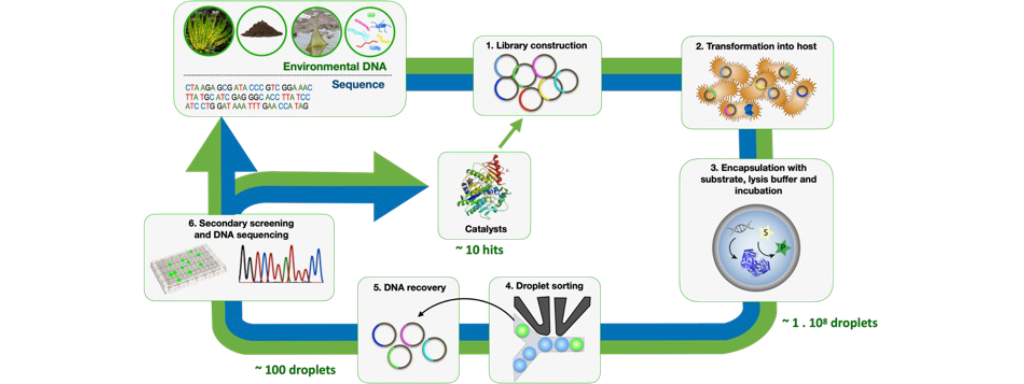
Since Francis Arnold won the Nobel Prize in 2018, directed evolution has rapidly gained recognition for its potential to generate new, highly efficient enzymes. The method is relatively simple: the desired reaction is chosen and a parent enzyme with some ability to catalyse this reaction is identified. The gene which corresponds to the parent enzyme is then mutated to generate a library of genes which encode slightly different enzymes. These genes are then put into a micro-organism, like a bacterium, and are used to produce new enzymes. These enzymes, which will have undergone structural and functional changes due to the mutations, are screened for their activity in catalysing the desired reaction, and the best mutant is chosen. The most active enzyme(s) can then be selected and used as parents for a further round of evolution
Figure one: The workflow of a typical directed evolution campaign, where eDNA or sequences from databases are screened and sorted into microdroplets for analysis of their activity. Successful candidates can be subjected to rounds of evolution to screen for improved activity.
Combining directed evolution and high-throughput microfluidics to develop plastic-degrading enzymes
Plastics are man-made materials, so finding a natural enzyme able to catalyse their degradation can be challenging. Typically, new enzymes for such processes have been discovered by analysing the DNA from bacteria and other microbes and studying the how the natural enzymes they produce are able to degrade different materials. However, when no natural enzymes are available for degrading a particular material, it may be necessary to use an enzyme which catalyses a similar reaction instead. Based on the plastics targeted in this project, natural small molecule esterases, amidases and urethanases could make appropriate parent enzymes for evolution.
A crucial element of screening for successful mutants is the assay used to characterise them. Typically, researchers use a modified version of the material they want to degrade (the substrate) that produces florescent light when it is degraded. This lets them measure the amount of fluorescence emitted and when more light is emitted this indicates that the enzyme is better at degrading the substrate. However, this can bias the evolution process towards improving activity for the modified version of the substrate rather than the substrate itself. In plastic degradation this is especially important as enzymes interact differently with high molecular weight polymers than with small molecules containing the same functional groups (such as esters or amides). To address this issue, the group has designed a patented ultrahigh-throughput (kHz) nanoparticle-based assay to ensure the evolution directly improves enzyme activity on the polymers. This also allows assessment of the enzymes’ ability to deal with different material properties of the plastics, such as particle sizes and crystallinity, which are otherwise hard to screen for.
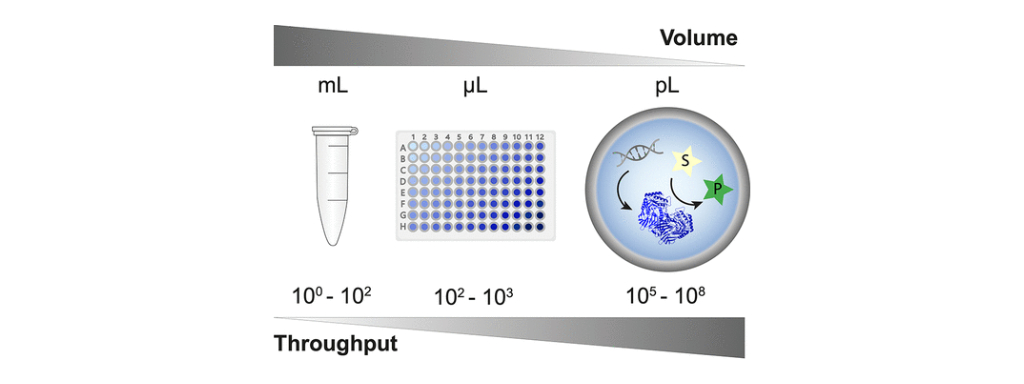
A screening method for a successful directed evolution campaign must also be fast, efficient and reliable. The pool of known, naturally occurring enzymes is vast and ever-increasing, and introducing mutations expands this pool exponentially. The Hollfelder group are experts in droplet microfluidics, a technique which can screen the activity of enzymes inside picolitre scale microreactors, minimising the time, cost and material requirements for identifying suitable enzymes. The ‘lab-on-a-chip’ designs allow the screening of up to 108 different variants per day, several orders of magnitude higher than other widely used techniques. Since microfluidics generates large data sets, it is also extremely powerful for training machine learning models. By collaborating with computational scientists through the P3EB Mission Hub, specifically Christine Orengo’s group at UCL, the plastic degrading activity of enzymes can be linked to their structural and sequence data. This allows the development of algorithms which identify distant relationships between proteins and can predict mutants with improved plastic degradation capabilities, creating a design-build-test-learn cycle which is central to the framework of synthetic biology and allows for rapid and efficient design of plastic-degrading enzymes.
Figure 2: How droplet microfluidics enables a massive scale-down of reaction volumes, from milliliters in test tubes, beyond microliters used in plate formats and robotic liquid handling systems to picoliters in in vitro compartments. The corresponding increase in throughput possible is shown.
Further Information
For further information on research in the Hollfelder group, including and beyond the P3EB project, please see our group website.
For further information on the use of droplet microfluidics for enzyme engineering, please see our Trends in Biochemical Sciences special issue article for a brief overview or our recent Chemical Reviews article for an in-depth review.
Author Information
Postdoctoral Research Associate
Hollfelder Group
Image Credits
All image credits: Hollfelder lab